
What idea of the atom did it suggest? (The nuclear model.) What model of the atom did this replace? (Thomson’s plum pudding model, in which atoms are seen as essentially small balls composed of a mixture of positive and negative electric charge, with no concentration of charge at any particular position.) Discussion: Models in physics (10 minutes)ĭiscussion: Recollecting the significance of Rutherford’s experimentĪs a preparatory task, ask your students to revise what they have previously learned about Rutherford’s α-scattering experiment.Question: Rutherford’s results (optional).Student questions: Rutherford experiment and atomic structure (optional) (20 minutes).Student experiment or demonstration: The Chinese hat analogue (30 minutes).Discussion: Rutherford scattering and Coulomb’s law (10 minutes).Demonstration: Collisions and momentum (10 minutes).Discussion: Rutherford’s experiment (20 minutes).Discussion: Recollecting the significance of Rutherford’s experiment (10 minutes).In this episode, students look in detail at Rutherford’s experiment and relate it to a mechanical analogue. This topic also provides a good opportunity to discuss the use of models in physics, including both mechanical and mathematical models.
.png)
Once the idea of the nuclear atom is established, you can go on to look at nuclear structure, particle accelerators, the Standard Model and the whole of particle physics. If you have not covered all of these topics already, you will have to modify the suggested approach to take account of this. There is a lot of Physics knowledge that can contribute to this topic: collisions and momentum, Coulomb’s law, and wave-particle duality.
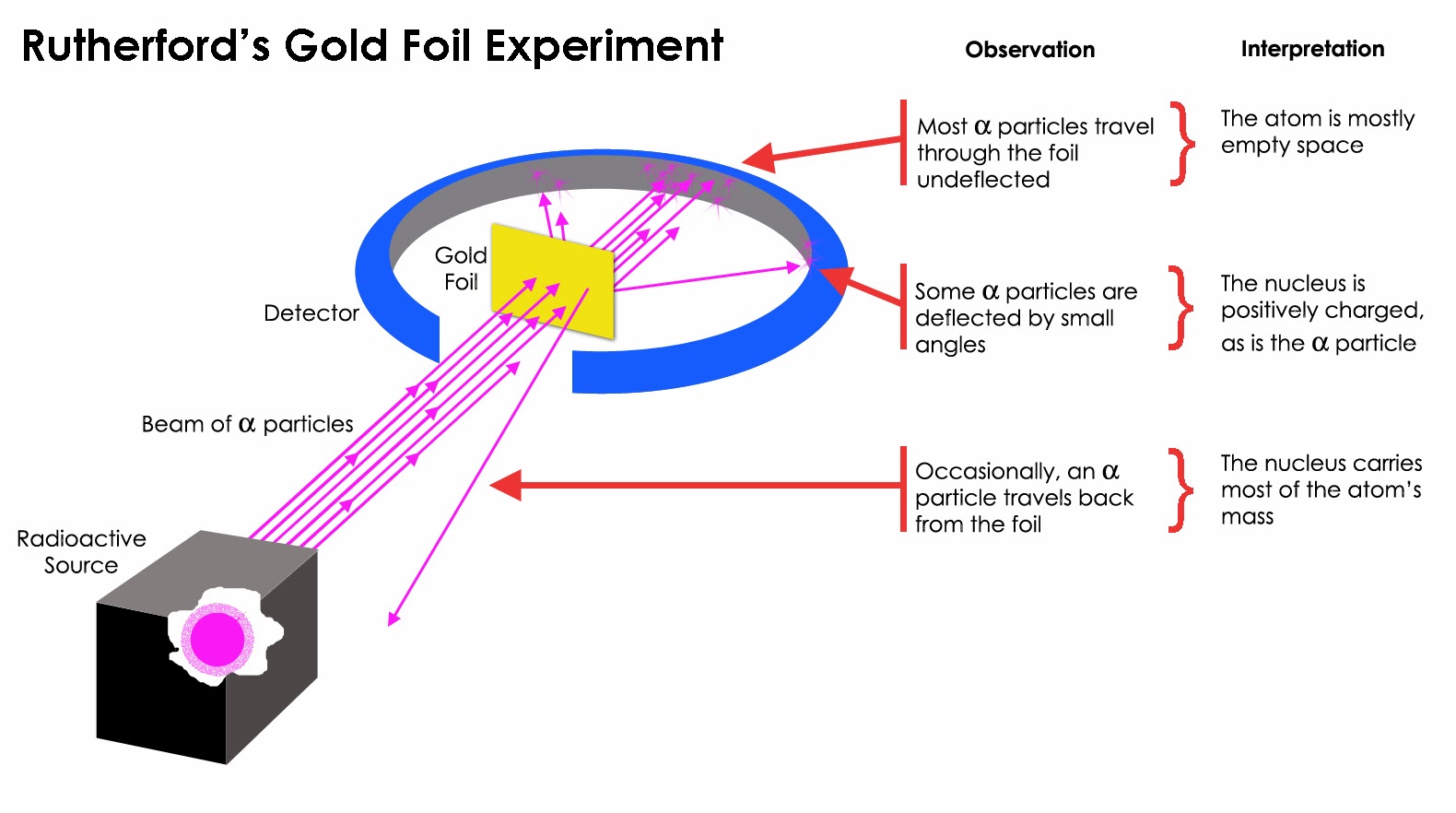
describe Rutherford’s experiment and explain why it leads to the nuclear model of the atom.Here you have the opportunity to deepen their understanding of the nuclear model of the atom, making use of ideas about electric fields.


 0 kommentar(er)
0 kommentar(er)
